Acorn ACN-101 Study Overview
Quantifying the real-world impact of Acorn’s autologous secretome.
ACN-101 is a multi-site, prospective observational study measuring how Acorn’s autologous hair follicle secretome (aHFS) supports post-procedure recovery, aesthetic results, and patient satisfaction across:

Face Microneedling
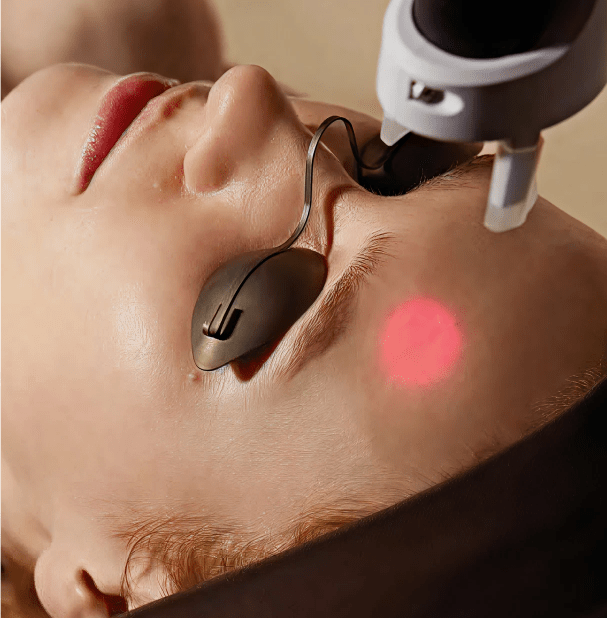
Laser Resurfacing
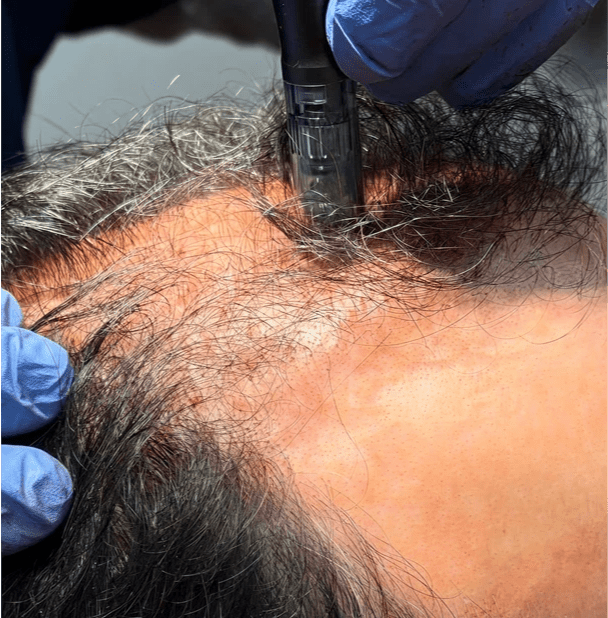
Scalp Microneedling
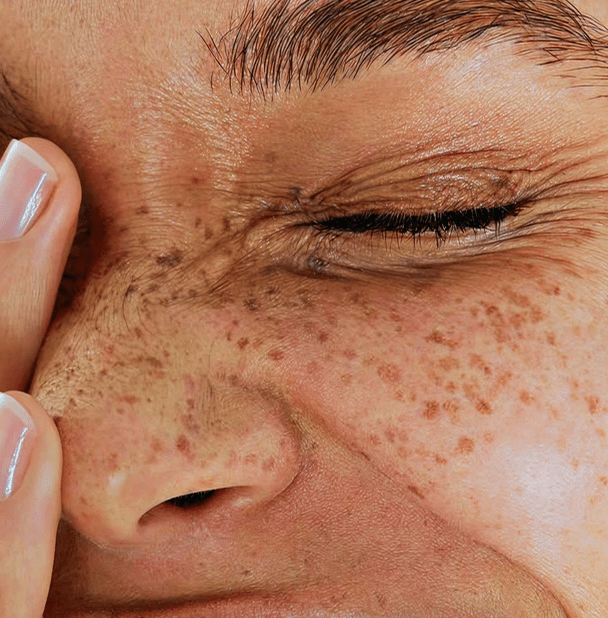
Scar Treatments
Unlike traditional clinical trials, participants paid
for both their procedures and the Acorn product, ensuring unbiased outcomes grounded in real-world use. Data was captured through electronic patient-reported outcomes (ePRO), physician assessments, and standardized 2D and VISIA imaging.
Study Design
30+ clinical sites across the U.S. and Canada with 121 patients enrolled (as of June 2025)
- Age: 18–64, with the majority between 40–55 years old
- Gender: 65% female, 35% male
Real-world setting
No placebos or free product
Double-blinded photo assessments
Follow-up from Day 7 through Day 365
The study is ongoing, with more data to come
How We Captured the Data
From Patients
Using a secure electronic platform (ePRO), participants reported on post-procedure symptoms, downtime, product impressions (like texture and scent), satisfaction, and perceived aesthetic improvement.

From Providers
Physicians collected standardized before-and-after photography, visual improvement ratings (e.g., GAIS), and quantitative skin or hair assessments.


Our Findings
Hero Claims
- 100%
tolerability with no product-related adverse events during a 14-week repeat insult patch test^
- 100%
of patients returned to baseline by Day 4**
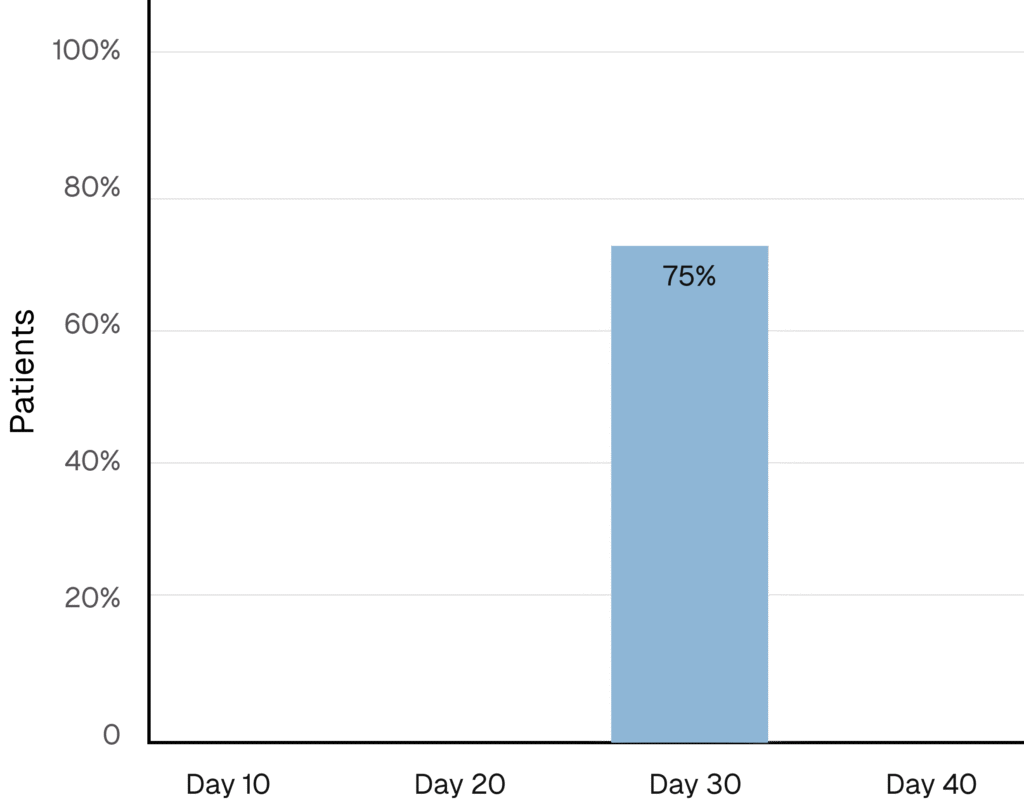
Practitioners Report Visible Results
75% of patients saw visible improvement in their skin by Day 30**
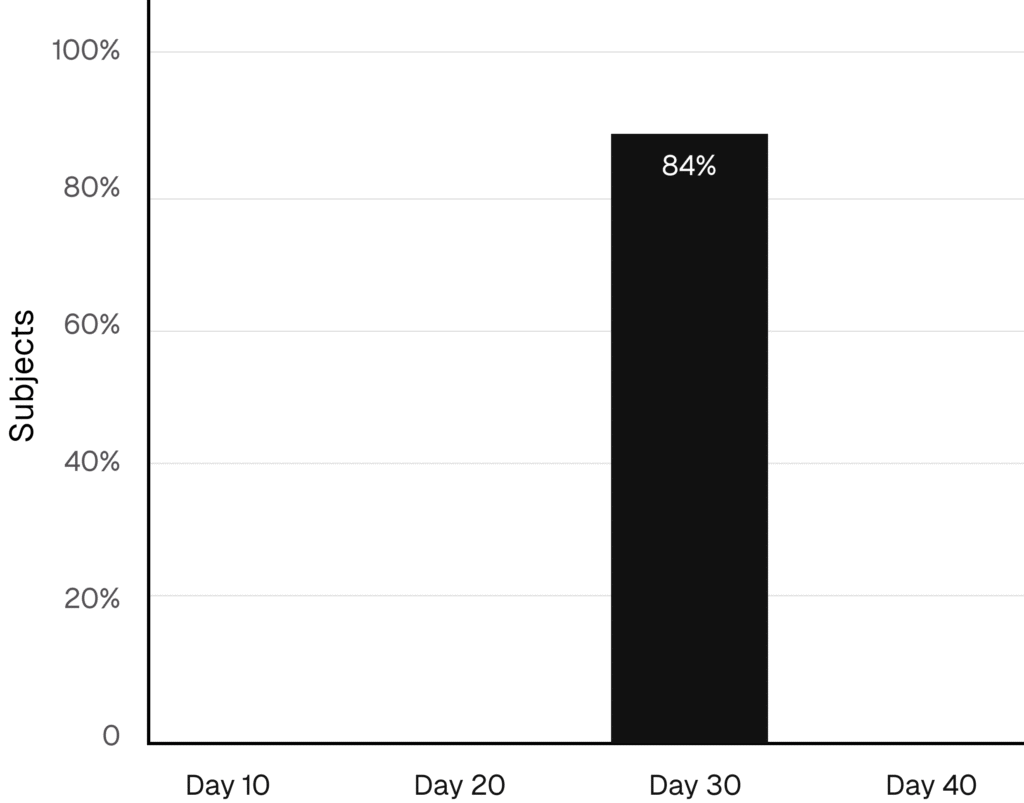
Patients See Visible Results
84% of subjects showed clear aesthetic improvement by Day 30, based on GAIS scores and photos confirmed**
94%+ of patients preferred Acorn’s autologous secretome over donor-derived exosomes, umbilical cord blood, plant-based exosomes, PRP/PRF made from their own blood****
Why?
The majority chose Acorn for “better experience”, “faster healing,” and “natural origin”
*n=27, microneedling only group
**n=32, microneedling + laser patient group combined, based on patients’ self-assessment
***n=36, microneedling group only (self + physician assessment)
****n=70, ePRO preference survey (self-reported, mixed group)
^ n=50, 14-week test for adverse reactions, no adverse events earning mark as “Dermatologist Tested/Approved”
- 91%
would recommend Acorn to others**
- 88%
would purchase the product again**
- 81%
felt it was worth the cost**
Designed to Inform What’s Next
This Study isn’t the End — It’s the Beginning.
ACN-101 was built to generate real-world insights, guide our clinical trials, and validate a new class of skin and hair treatments rooted in your own biology.